Common Specifications for Certain Class D IVDs
Commission Implementing Regulation (EU) 2022/1107 has been published and will come into effect on 25 July 2022 to lay down the Common Specifications for the following Class D IVDs with respect to requirements regarding performance characteristics as detailed in Sections 9.1(a-b), 9.3, 9.4(a) of Annex I of the EU IVD Regulation 2017/746:
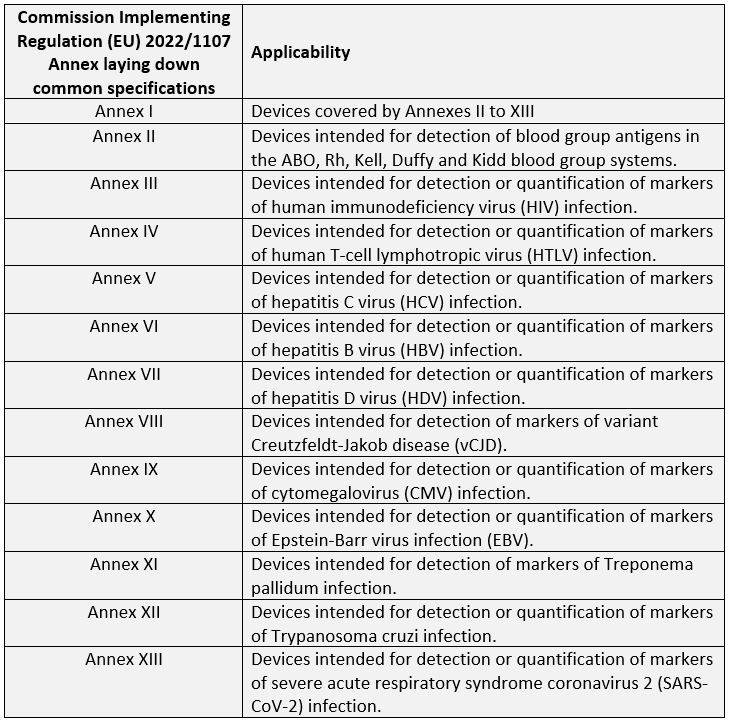
Common Specifications are a set of technical and/or clinical requirements that provide a means by which a product may comply with the legal obligations applicable to that device; however, this should not be confused with standards.
See the following link to access the full document detailing the Common Specifications: EUR-Lex – 32022R1107 – EN – EUR-Lex (europa.eu)

