We understand that the regulatory landscape of the MDR and IVDR is constantly changing, and in order to keep up to date with the changes, we have provided a current state of play with regards to some of the most important changes to the MDR and IVDR.
MDR Extension
Firstly, we would like to remind our clients of the MDR extension which came into force in March of last year. To offer some background to this extension, the EU commission proposed an extension in the transition periods due to concerns of medical device shortages on the EU market as a result of medical device manufacturers not having enough time to comply with the MDR requirements. The proposal faired quite well and was adopted and published in the official journal of the EU on the 20th of March 2023 and can be accessed using the following link (EU) 2023/607
Below, are the new deadlines that manufacturers of certain medical devices will have to be compliant with the MDR based on their classification.
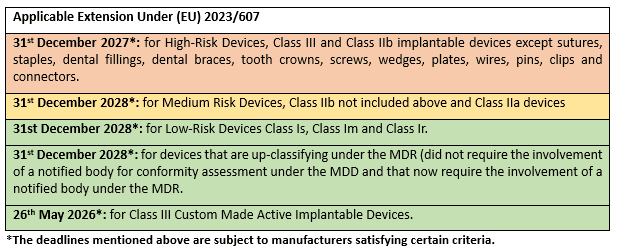
These deadlines mentioned above are subject to a number of criteria that the manufacturer must satisfy. One of these criteria, pertaining to the signing of a notified body agreement for the conformity assessment of their device under the MDR, had a deadline that recently passed of 26th September 2024. We hope that manufacturers that intended to make use of this extension have met this recent deadline.
Advena had released a guidance document that helps manufacturers navigate the complexities of this new regulation. The guidance document can be accessed using the following link
IVDR Extension
In a similar fashion, a comparable extension to transition periods was adopted for the IVDR. Again, this extension to the transition period was mainly fuelled by concerns of IVD shortages on the market due to IVD manufacturers not having enough time to comply with the IVDR requirements. In the view of the EU Commission, this extension will help to mitigate the risk of shortages of IVDs by giving manufacturers and notified bodies more time, provided certain conditions are met, to complete the necessary conformity assessment procedures, without lowering the requirements. The proposal faired quite well and was adopted and published in the official journal of the EU on the 9th of July 2024 and can be accessed using the following link (EU) 2024/1860.
Under the old legislation (EU) 2022/112, devices that had an IVDD certificate issued prior to 26th May 2022, would continue to be valid up until 26thMay 2025. Under the new legislation, this has been extended up until 31st December 2027.
In addition, under the old legislation, devices that were considered general IVD’s under the IVDD and need the involvement of a notified body for conformity assessment under the IVDR, would have an extension in transitional period depending on the classification under the IVDR. The extension in the transitional period was as follows: May 2025 for High-Risk Class D devices, May 2026 for Medium Risk Class C devices, and May 2027 for Low-Risk Class A in a sterile condition and Class B devices.
Under the new legislation, manufacturers would be given an extra 2 years and a half to fully comply with the IVDR requirements when compared to the old legislation. This means that the extension in the transitional period under this new legislation is as follows: December 2027 for High-Risk Class D devices, December 2028 for Medium Risk Class C devices, and December 2029 for Low-Risk Class A in a sterile condition and Class B devices
The table below highlights the old deadlines and the newly proposed deadlines for the different classes of IVD devices.
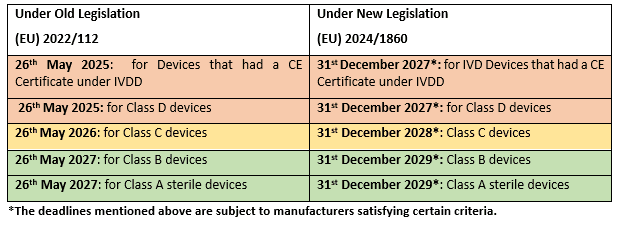
These deadlines mentioned above are subject to a number of criteria that the manufacturer must satisfy. Please also be aware that some of the criteria have certain deadlines based on the classification of the device under the IVDR.
Advena had released a guidance document that helps manufacturers navigate the complexities of this new regulation. The guidance document can be accessed using the following link
Gradual Roll-out of EUDAMED
The EU commission had also proposed the gradual rollout of EUDAMED where modules would be audited, tested and subsequently made available separately, hence removing the reliance of the other modules. This proposal was also well received and was published in the OJEU on 9th of July 2024.
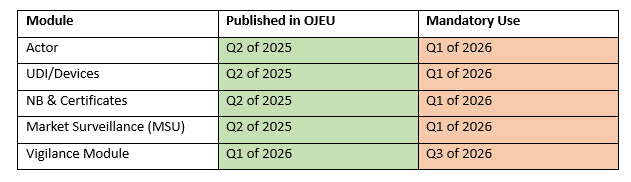
To further clarify their plan moving forward, the EU Commission published a roadmap to demonstrate how the gradual rollout of EUDAMED would work in practise. This roadmap is intended to give the relevant actors estimated timeframes of when each module will become mandatory for use.
According to the roadmap published, the notice for the Actor, UDI/Devices, NB & Certificates and Market Surveillance (MSU) Modules will be published in the OJEU in Q2 of 2025. This means that the mandatory use of these modules will be effective 6 months from this date in Q1 of 2026.
Furthermore, the notice for the vigilance module will be published in Q1 of 2026, hence meaning that this module will become mandatory as from Q3 of 2026.
Obligation of Manufacturers to inform the relevant actors if they intend to cease supply of a critical medical device
Lastly, (EU) 2024/1860 introduces an additional obligation for manufacturers to inform the relevant actors if they intend to cease supply of a critical medical device. To offer some background to this amendment, during the transitional period, many actors such as healthcare professionals and competent authorities have reported that the supply of many medical devices and IVDs has stopped, or likely to be stopped. If the medical device is a vital medical device, the interruption of supply can result in serious harm or a risk of serious harm to patients or public health. The amendment aims to impose an additional obligation to the manufacturers making them inform the relevant actors if they intend to cease, temporarily or permanently, supply of a critical medical device, hence enabling the authorities to carry out the necessary mitigating measures to ensure public health and safety. Under this new legislation, manufacturers will be required to inform the relevant actors 6 months prior to anticipated interruption to the supply of a critical medical device on the EU market.

