Manufacturers of in vitro diagnostic medical devices (IVDs) under Regulation (EU) 2017/746 (IVDR) must be aware of a fast-approaching regulatory milestone.
In accordance with (EU) 2024/1860, in order to benefit from the extended IVDR transition periods, manufacturers must satisfy a number of criteria, two of which will be due at the end of May. These include:
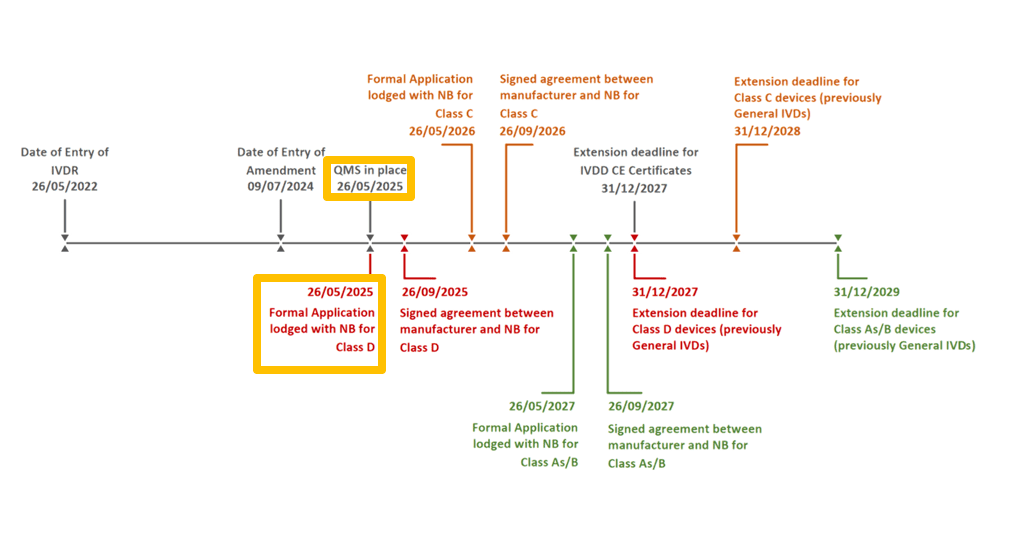
- QMS Implementation: Implementing a QMS in accordance with Article 10(8) of the IVDR by 26th May 2025.
- Submitting a formal application: For Manufacturers of Class D IVD devices, a formal application to a Notified Body for the conformity assessment of their devices must be submitted by 26th May 2025.
Failure to meet this deadline will mean losing access to the extended transition timelines, and manufacturers would be prohibited from placing affected devices on the EU market after their current certificates expire or — for legacy devices — beyond the original IVDR dates.
Why This Deadline Is So Important?
The European Commission introduced staggered transition periods to give manufacturers more time to comply with the stricter requirements of the IVDR. Based on the classification of the device under the IVDR, manufacturers would have additional time to comply with the requirements of the IVDR, the extensions that will be granted to the different classifications can be seen below;
- 31st December 2027, for IVD Devices that had a CE Certificate under IVDD*
- 31st December 2027 for Class D devices*
- 31st December 2028 Class C devices*
- 31st December 2029 Class B devices*
- 31st December 2029 Class A sterile devices*
Conditions that Need to be Met
There are a number of conditions that need to be met for the manufacturer to be eligible for the extensions in the transitional periods. some of these conditions are classification sensitive while some others apply to all classifications at the same time.
Classification Sensitive Deadlines
These extensions are conditional — they only apply if manufacturers take proactive steps, including:
- Having a formal application for conformity assessment submitted to a Notified Body
- by 26th May 2025: for devices having a risk classification of Class D
- by 26th May 2026: for devices having a risk classification of Class C
- by 26th May 2027: for devices having a risk classification of Class B
- by 26th May 2027: for devices having a risk classification of Class A in a Sterile Condition
- Ensuring that a written agreement with the Notified Body is in place no later than
- by 26th September 2025: for devices having a risk classification of Class D
- by 26th September 2026: for devices having a risk classification of Class C
- by 26th September 2027: for devices having a risk classification of Class B
- by 26th September 2027: for devices having a risk classification of Class A in a Sterile Condition
General Conditions
- The devices continue to comply with Directive 98/79/EC (IVDD).
- There are no significant changes in the design and intended purpose.
- The devices do not present an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the protection of public health.
- No later than 26th May 2025, the manufacturer has put in place a quality management system in accordance with Article 10(8).
- No later than 26th September the Notified Body who issued CE Certificate under the IVDD to sign agreement with respect to the surveillance of device
Without meeting these conditions, the device must fully comply with the IVDR immediately after 26 May 2025, without benefiting from additional transition time.
Last year, Advena had published a guidance document in relation to (EU) 2024/1860 to help manufacturers understand the amendment to the regulation. The guidance document can be accessed using the following link.
Important Deadlines for the Extension in the Transitional Period for IVD Manufacturers of Different Classes