The European Commission has released an updated version of the Manufacturer Incident Report (MIR) form (Version 7.3.1), which was previously last revised in 2019. This form serves as a standardized template that manufacturers can use to report incidents involving medical devices to the relevant competent authorities. The New MIR form becomes mandatory as from November 2025.
Key Updates to the MIR Form:
Section 1.2
Section 1.2 now includes the field “Manufacturer awareness date of reportability”, which refers to the date the manufacturer received information indicating the incident is reportable.

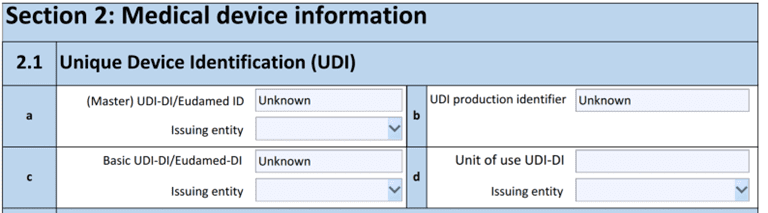
Section 2.1
Section 2.1 introduces dropdown options to specify the UDI issuing entity, where applicable.
Section 2.4
Section 2.4 adds new fields to indicate the following:
- If the applicable legislation is unknown

- Whether the device continued to be placed on the market after the date of application of the MDR or IVDR

- Whether any of the following provisions apply: MDR Article 52(9), MDR Article 52(10), IVDR Annex IX Section 5.2, or IVDR Annex X Section 3.k.

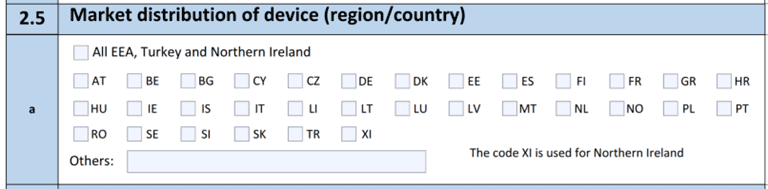
Section 2.5
Section 2.5 has been updated to remove Switzerland and add Northern Ireland to the list of regions.
Section 4.1
Section 4.1 now includes a new question:
- “Is there a suspicion of a relationship between the incident and any associated medicinal substance(s)/product(s), tissue(s), cell(s) of human origin, or their derivatives

The new MIR Template can be accessed using the following link

