The EU Commission has updated the current state of MDR/IVDR applications with the notified bodies. This new update is concerning a period ending in October 2024. The results have been documented in a .pdf power point which is split up into 4 sections:
- About the study and survey.
- Survey results for medical devices.
- Survey results for in vitro diagnostic medical devices.
- Staff
MDR Statistics
The MDR survey results concerning a period ending on October 2024 show that there has been a total of 28,069 MDR applications submitted and 10,554 MDR certificates issued. Comparing these figures to the figures that have been reported in June, these latest statistics show an increase of 2,082 (or 8%) in the number of MDR applications submitted and an increase of 1,654 (or 19%) in the number of MDR certificates granted.
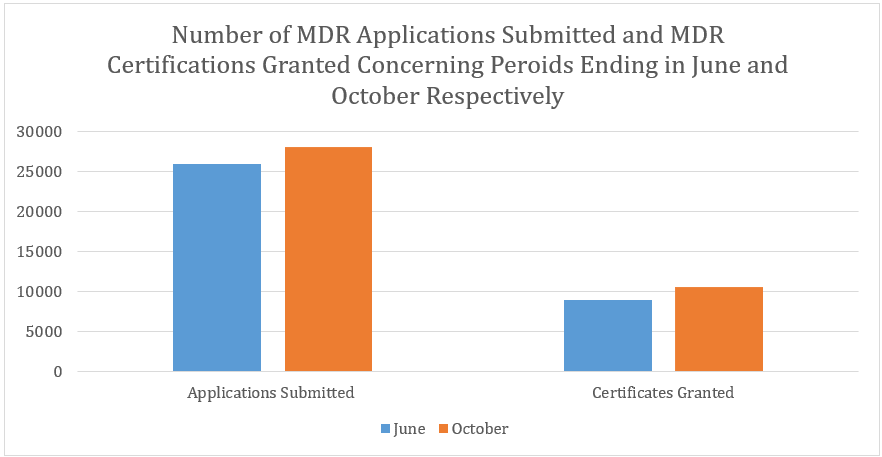
IVDR Statistics
The IVDR survey results concerning a period ending on October 2024 show that there has been a total of 2,201 IVDR applications submitted and 1,273 IVDR certificates issued. Comparing these figures to the figures that have been reported in June, these latest statistics show an increase of 454 (or 26%) in the number of IVDR applications submitted and an increase of 333 (or 35%) in the number of IVDR certificates granted.
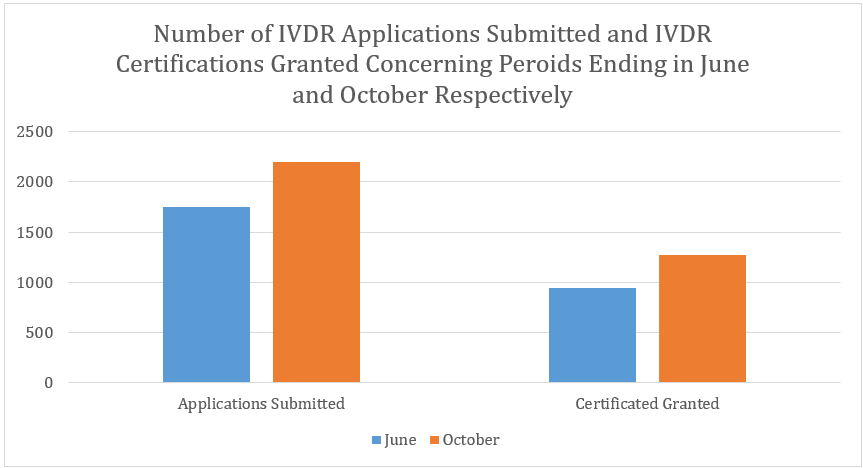
Key Takeaway for Manufacturers
These numbers confirm the continuing high demand for MDR and IVDR certification services. If you have not yet initiated your certification process, we strongly recommend that you begin as soon as possible to avoid potential delays and bottlenecks.
Given the limited capacity of larger Notified Bodies, we also encourage manufacturers to consider smaller or lesser-known Notified Bodies, which may have more availability and be able to process your application more quickly.
The full results from the survey can be downloaded using the following link

