In accordance with the new EU Regulations, the Medical Device Regulation 2017/745 (MDR) and the EU IVD Regulation 2017/746 (IVDR), manufacturers must assign a Basic UDI-DI (BUDI) to each of their devices (apart from custom-made devices). The Basic UDI-DI is an alphanumeric code assigned by the manufacturer which indicates a particular device group and is defined as:
the primary identifier of a device model. It is the DI assigned at the level of the device unit of use. It is the main key for records in the UDI database and is referenced in relevant certificates and EU declarations of conformity.
Unlike the UDI Carrier, the BUDI is not displayed on the device, its labelling, or packaging, however, it will be indicated within the technical documentation, applicable certificates, and the Declaration of Conformity as a minimum.
A UDI-DI can only be associated with one Basic UDI-DI; thus, in Eudamed multiple UDI-DI registrations will be linked to a single Basic UDI-DI.
What is the difference between a Basic UDI-DI and a UDI-DI?
We must agree, the terminology used doesn’t help! However, the Basic UDI-DI is primary alphanumeric identifier of a group of devices with the same intended purpose, classification, essential design and manufacturing characteristics, which does not appear on the labelling. Think of this as the “root” identifier.
On the other hand, the UDI-DI is a numeric identifier which refers to a specific product. Therefore, investigating a UDI-DI will lead you to one particular device variant, whilst a Basic UDI-DI may lead you to many UDI-DI.
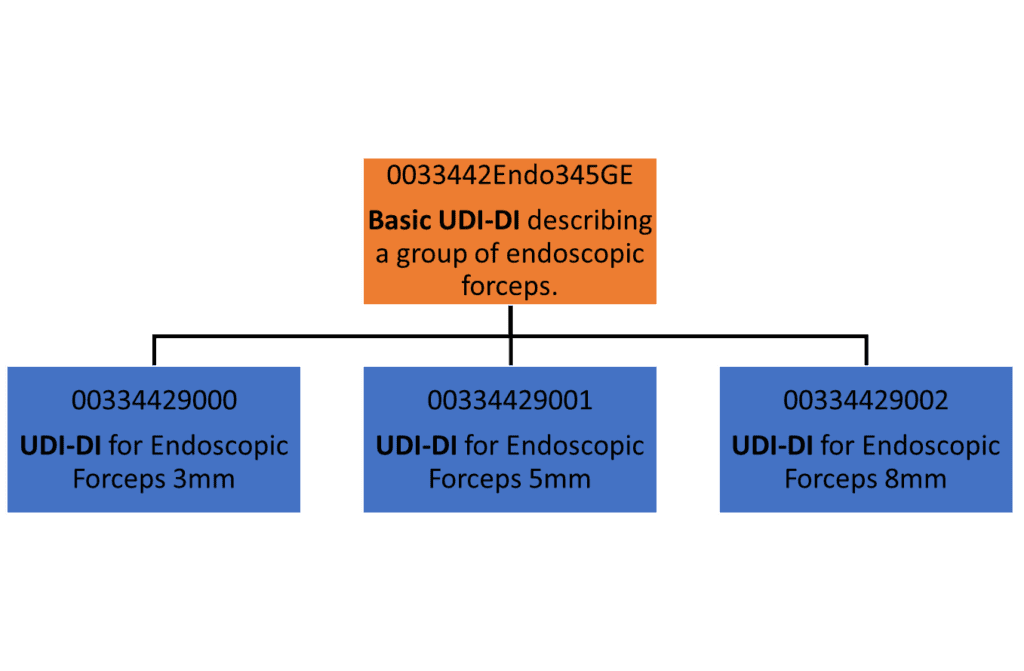
Image is not based on any particular UDI-issuing entity standards.
How do I assign the Basic UDI-DI?
The first step will be to split up your devices into different groups which will then be assigned a BUDI. The Regulation does not provide any specific guidance as to how this should be done, however, MDCG 2018-1 provides a hint that the BUDI is intended to connect devices with same intended purpose, risk classification and essential design and manufacturing characteristics. To get more information on how to group devices under a different BUDI, we may also look at the structure of Eudamed registrations.
Based on this guidance and the Basic UDI-DI Registration structure on Eudamed, we can recommend the following scheme for hierarchy for grouping your devices:
Level 1: Intended Purpose
Only devices with the same intended purpose shall be listed under a single Basic UDI-DI. This includes a consideration of indications, contra-indications, target users and target patients.
Level 2: Classification
Devices with different classifications shall be separated into a different Basic UDI-DI.
Level 3: Design Characteristics
The design of devices under a single BUDI grouping should have the same essential design characteristics. One example is when a product with the same intended use and classification can be provided active or not active (e.g. hot vs cold endoscopic instruments); these should have two separate BUDIs. Another example may be one in which a variant has a measuring function.
Level 4: Manufacturing Characteristics
Devices with different manufacturing characteristics shall be assigned a different BUDI. In some cases, the same device may be provided as sterile and non-sterile; these should have separate BUDIs.
Until more formal guidance is released, we recommend the use of MedTech Europe’s guidance document for assigning Basic UDI-DI which is very comprehensive.
Basic UDI-DI Anatomy and Validation
The BUDI is typically formed from 3 main elements: Company Prefix, User-Inputted Alphanumeric Code, Check Characters.
GS1
GS1 refer to the BUDI as the Global Model Number (GMN), which is structured using the GS1 Company Prefix (acquired upon registering with GS1), the user-inputted alphanumeric code, and automatically generated check characters:

You may generate/validate the GMN using the resource at the following link: https://www.gs1.org/services/check-character-calculator
HIBCC
The HIBCC Basic UDI-DI is generated using the organisation’s Labeler Identification Code (LIC) (acquired upon signing up with HIBCC), and automatically generated check characters. HIBCC BUDI typically contain a ‘++’ prefix, e.g. ++A999MODELIDENTIFIER11S8.
You may generate/validate the HIBCC BUDI using the resource at the following link: https://www.hibcc.org/basic-udi-di-generator/
ICCBBA
At the time of writing of this article, there is generator for ICCBBA BUDI. However, you may find more information on how to generate it at the following link: https://ec.europa.eu/docsroom/documents/38567?locale=en
ICCBBA are 18 characters long, with two of these acting as the ‘checksum’.

