A label provides a wealth of information regarding your medical device that is communicated to the end user or patient. Therefore, it is of utmost importance that you have all the relevant information on the label as laid out in Annex I Section 23 of the MDR.
Annex I 23.1 (h) states that;
Where appropriate, the information supplied by the manufacturer shall take the form of internationally recognised symbols. Any symbol or identification colour used shall conform to the harmonised standards or CS. In areas for which no harmonised standards or CS exist, the symbols and colours shall be described in the documentation supplied with the device.
What this essentially means is that symbols that are used on the label should be from a harmonized standard. In an attempt to avoid any barriers of communication between different member states of the union, the MDR encourages manufacturers to use symbols that are internationally recognized. This would ultimately save the hassle of translating every item on the label. The standard that we recommend using is ISO 15223-1 which is a standard specifically created for symbols used on the packaging of medical devices.
Annex I (23.2) of the MDR guides you to what information needs to be on the label. Below we will discuss some of the most important details that you would need on your label (please note that the list provided below is not exhaustive and your device might require additional information to be MDR compliant)
23.2 (c) the name, registered trade name or registered trademark of the manufacturer and the address of its registered place of business;
This point states that the address of the legal manufacturer should be placed on the label. If you engage with us, this should be the address given to us. The address is usually preceded by the following symbol from the ISO 15223-1 standard as shown below;

23.2 (d) if the manufacturer has its registered place of business outside the Union, the name of the authorized representative and address of the registered place of business of the authorized representative.
This point states that if your company is situated outside of the union, then the name and details of the authorized representative should be on the label. This is an important aspect of the label as the EC REP is the main contact point within the Union. The address is usually preceded by the following symbol from the ISO 15223-1 standard:

23.2 (h) the UDI carrier referred to in Article 27(4) and Part C of Annex VI;
This requirement states that the UDI carrier should be placed on the label according to the dates below. Depending on the classification of the device, the label has a certain deadline to comply with these requirements. The deadlines are listed below:
- Implantable and Class III – 26 May 2021
- Class IIa and IIb (non-implantable) – 26 May 2023
- Class I – 26 May 2025
The following symbol in accordance with the ISO 15223-1 standard can be used followed by the UDI carrier;

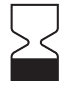
23.2 (i) an unambiguous indication of t the time limit for using or implanting the device safely, expressed at least in terms of year and month, where this is relevant;
As the above point states, the time limit in which the device could be used safely should be communicated to the user. This should be in terms of the year and month, however we do suggest adding the day also. The following symbol from the ISO 15223-1 standard is usually used followed by the date of expiry of the device.
23.2 (j) where there is no indication of the date until when it may be used safely, the date of manufacture. This date of manufacture may be included as part of the lot number or serial number, provided the date is clearly identifiable;
This point states that the date of manufacture should be placed on the label. This would usually be preceded by the following symbol;

Sometimes, manufacturers like to display where the product has been manufactured on the label. This could be combined with the symbol above, where the country of manufacture is placed inside the symbol using a 2-letter code from the ISO 3166-1 standard. Let’s say that your product was manufactured in Brazil, then you would replace the CC 2 letter code to BR to represent Brazil.

Thae date of manufacture could also be added to the lot or serial number in a way that is recognized by the user. This would be a clear code, usually at the end of the number in the format DD-MM-YYYY.
For example, XXXXXXXXXXXX26122022, indicating that the product was manufactured on the 26th of December 2022.
23.2 (q) an indication that the device is a medical device. If the device is intended for clinical investigation only, the words ‘exclusively for clinical investigation’;
This point indicates that the manufacturer should place the MD symbol on the labelling of the device which communicates to the user that the device is a medical device. The following symbol from the ISO 15223-1 standard is usually used;

Lastly manufacturers are required to affix the CE marking with the correct proportions on the label of the medical device. The correct proportions could be found using the following Link.

It is important for manufacturers to understand how to label their devices in accordance with the requirements of the MDR as non-conformities with regards to labelling could lead to your product being held up at customs and cause considerable delays!

